Audit of crystallisation incidents where calcium folinate is administered concurrently with SACT
Caroline Clapham Bedfordshire Hospitals NHS Foundation Trust
Maryam Rasoolirad Bedfordshire Hospitals NHS Foundation Trust
Background
Calcium folinate forms part of multiple systemic anti-cancer therapy (SACT) regimens. It is common practice to administer calcium folinate via a ‘Y-site’ connector concurrently with irinotecan or oxaliplatin.
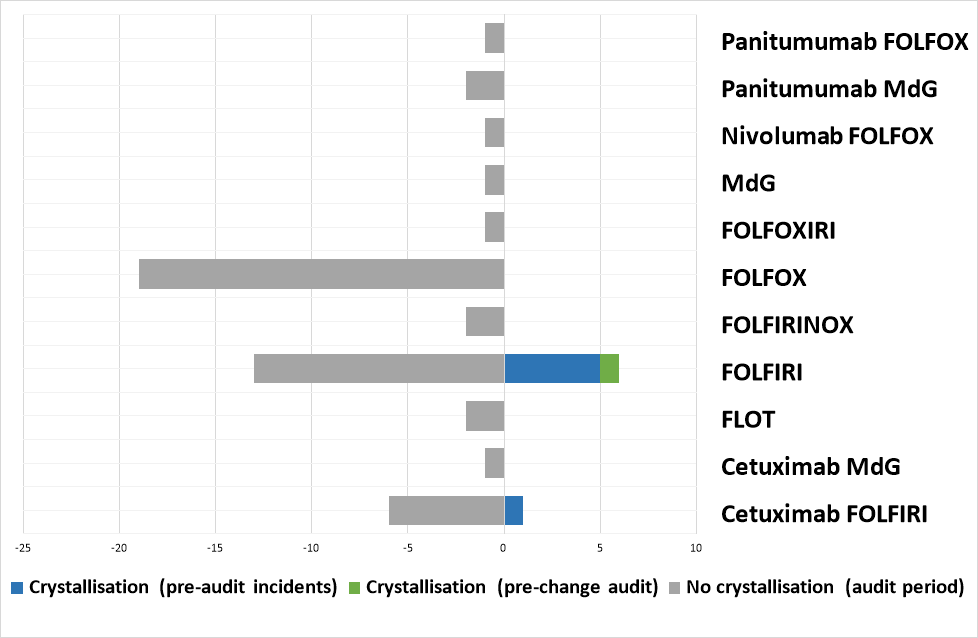
Between August and November 2023, there were 7 reports of crystallisation in the giving sets of patients receiving FOLFIRI on the Primrose Oncology Unit at Bedford Hospital, with no previous reported incidents. An audit was conducted to investigate.
Objectives
- To ascertain the incidence of crystallisation when administering with SACT via Y-site
- To ascertain the point during treatment where crystallisation occurs
- To assess the success of implementation of separate administration of calcium folinate
Method
An audit tool was designed to enable nursing staff to assess the patient’s line for crystallisation after each drug administration. Data collection took place over a period of 2 weeks during November 2023. All patients receiving a SACT regimen during the audit period in which calcium folinate is administered concurrently with SACT were included.
Following preliminary analysis, it was decided that irinotecan and calcium folinate would be administered separately. A post-change re-audit was then completed over a period of 1 week to ensure crystallisation events had been eliminated.
Results
During the initial audit period, 35 patients received calcium folinate containing SACT regimens, including 11 FOLFIRI patients. Of these, crystallisation occurred in the line of 1 patient, after the administration of the irinotecan and calcium folinate (2.86% of all audited patients, 9.1% of FOLFIRI patients).
Following the implementation of separate calcium folinate and irinotecan administration, 15 patients were audited during the re-audit period, including 9 FOLFIRI patients. The was no incidence of crystallisation during the re-audit period (0% of all patients)
![]()

Discussion
From the data available, this author believes the most likely cause of the crystallisation was due to an incompatibility between the calcium folinate and irinotecan, as all incidents occurred in FOLFIRI patients and, during the audit, the crystallisation was observed following the administration of irinotecan and calcium folinate. However, as no chemical analysis had been conducted, it is difficult to draw definitive conclusions.
Since moving to separate administration of irinotecan and calcium folinate, 1 crystallisation incident has been reported due to concurrent administration of irinotecan and calcium folinate as a result of non-compliance with the new policy.
It is not clear whether the trometamol-containing brand of calcium folinate, recently discussed by Polwart et al1 contributed to these events. Although the brand of calcium folinate used in the Trust at the time does contain trometamol (an excipient that is documented as being incompatible with oxaliplatin).
As no crystallisation events were seen in patients receiving oxaliplatin, there are no plans to move to separate administration of oxaliplatin and calcium folinate at Bedford Hospital.
Conclusion
Separate administration may be the safest option for administration of calcium folinate with SACT, until further data is available to support concurrent administration.
References
For more information:
Audit template: